Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\)
\(m_{CuCl_2}=270.10\%=27\left(g\right)\Rightarrow n_{CuCl_2}=\dfrac{27}{135}=0,2\left(mol\right)\)
Ta có: \(\dfrac{0,15}{1}< \dfrac{0,2}{1}\) ⇒ Fe hết, CuCl2 dư
PTHH: Fe + CuCl2 ---> FeCl2 + Cu
Mol: 0,15 0,15 0,15 0,15
\(a=m_{Cu}=0,15.64=9,6\left(g\right)\)
b, \(m_{dd.sau.pứ}=8,4+270-9,6=268,8\left(g\right)\)
\(m_{CuCl_2dư}=\left(0,2-0,15\right).135=6,75\left(g\right)\)
\(\left\{{}\begin{matrix}C\%_{CuCl_2dư}=\dfrac{6,75.100\%}{268,8}=2,51\%\\C\%_{FeCl_2}=\dfrac{0,15.127.100\%}{268,8}=7,09\%\end{matrix}\right.\)
c, \(V_{ddCuCl_2}=\dfrac{270}{1,35}=200\left(ml\right)=0,2\left(l\right)\)
\(\left\{{}\begin{matrix}C_{M_{CuCl_2dư}}=\dfrac{0,2-0,15}{0,2}=0,25M\\C_{M_{FeCl_2}}=\dfrac{0,15}{0,2}=0,75M\end{matrix}\right.\)

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\\ m_{HCl}=\dfrac{109,5\cdot10\%}{100\%}=10,95\left(g\right)\\ \Rightarrow n_{HCl}=\dfrac{10,95}{36,5}=0,3\left(mol\right)\\ a,PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\\ \text{Vì }\dfrac{n_{Mg}}{1}< \dfrac{n_{HCl}}{2}\text{ nên sau p/ứ }HCl\text{ dư}\\ \Rightarrow n_{H_2}=0,1\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,1\cdot22,4=2,24\left(l\right)\)
\(b,n_{MgCl_2}=0,1\left(mol\right)\\ \Rightarrow m_{CT_{MgCl_2}}=0,1\cdot95=9,5\left(g\right)\\ m_{H_2}=0,1\cdot2=0,2\left(mol\right)\\ m_{dd_{MgCl_2}}=2,4+109,5-0,2=111,7\left(g\right)\\ \Rightarrow C\%_{MgCl_2}=\dfrac{9,5}{111,7}\cdot100\%\approx8,5\%\)

\(n_{HCl}=\dfrac{10\%.109,5}{36,5}=0,3\left(mol\right);n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\\ a,PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\\ Vì:\dfrac{0,3}{2}>\dfrac{0,1}{1}\Rightarrow HCldư\\ n_{H_2}=n_{MgCl_2}=n_{Mg}=0,1\left(mol\right)\\ n_{HCl\left(p.ứ\right)}=2.0,1=0,2\left(mol\right)\Rightarrow n_{HCl\left(dư\right)}=0,3-0,2=0,1\left(mol\right)\\ V_{H_2\left(đkc\right)}=24,79.0,1=2,479\left(l\right)\\ b,ddA:HCl\left(dư\right),MgCl_2\\ m_{ddA}=2,4+109,5-0,1.2=111,7\left(g\right)\\ C\%_{ddHCl\left(dư\right)}=\dfrac{0,1.36,5}{111,7}.100\%\approx3,268\%;C\%_{ddMgCl_2}=\dfrac{0,1.95}{111,7}.100\%\approx8,505\%\)

fe + cuso4 ---> cu + feso4
nfe=0,035, CMcuso4=(10*10*1.12)/160=0,7, ncuso4=0,07
nfe=0,035 < ncuso4=0,07 ===> cuso4 dư
dd gồm có feso4, cuso4 dư
CMcuso4dư=(0,07-0,035)/0.1=0.35M
CMfeso4=0,035/0,1=0,35M

a) \(n_{Fe}=\dfrac{2,8}{56}=0,05\left(mol\right)\)
PTHH: `Fe + 2HCl -> FeCl_2 + H_2`
0,05->0,1----->0,05---->0,05
`=> V_{ddHCl} = (0,1)/2 = 0,05 (l)`
b) `V_{H_2} = 0,05.22,4 = 1,12 (l)`
c) `C_{M(FeCl_2)} = (0,05)/(0,05) = 1M`

Câu 1
\(a)PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\\
b)200ml=0,2l\\
n_{HCl}=0,2.1=0,2mol\\
n_{H_2}=n_{MgCl_2}=\dfrac{1}{2}n_{H_2}=\dfrac{1}{2}\cdot0,2=0,1mol\\
V_{H_2}=0,1.24,79=2,479l\\
c)C_{M_{MgCl_2}}=\dfrac{0,1}{0,2}=0,5M\)

a) \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
0,4-->0,6---------->0,2------->0,6
=> \(C_{M\left(dd.H_2SO_4\right)}=\dfrac{0,6}{0,15}=4M\)
b) VH2 = 0,6.22,4 = 13,44 (l)
c) \(C_{M\left(Al_2\left(SO_4\right)_3\right)}=\dfrac{0,2}{0,15}=\dfrac{4}{3}M\)
\(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\\
pthh:2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,4 0,6 0,2 0,6
\(C_M_{H_2SO_4}=\dfrac{0,6}{0,15}=4M\\ V_{H_2}=0,622,4=13,44L\)
\(C_M=\dfrac{0,2}{0,15}=1,3M\)


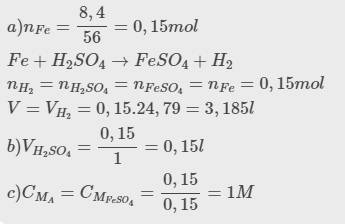

\(n_{Fe}=\dfrac{8,4}{56}=0,15mol\)
\(m_{CuCl_2}=\dfrac{270\cdot10\%}{100\%}=27g\Rightarrow n_{CuCl_2}=0,2mol\)
\(Fe+CuCl_2\rightarrow FeCl_2+Cu\)
0,15 0,2 0,15 0,15
\(a=m_{Cu}=0,15\cdot64=9,6g\)
\(m_{FeCl_2}=0,15\cdot127=19,05g\)
\(m_{ddFeCl_2}=8,4+270-0,15\cdot64=268,8g\)
\(C\%=\dfrac{19,05}{268,8}\cdot100\%=7,09\%\)