Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{CaCO_3}=\dfrac{20}{100}=0,2\left(mol\right)\\ a,PTHH:CaCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Ca+CO_2+H_2O\\ b,n_{CO_2}=n_{\left(CH_3COO\right)_2Ca}=n_{CaCO_3}=0,2\left(mol\right)\\ b,V_{CO_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\\ c,m_{\left(CH_3COO\right)_2Ca}=0,2.158=31,6\left(g\right)\\ d,C_4H_{10}+\dfrac{5}{2}O_2\rightarrow2CH_3COOH+H_2O\\ n_{C_4H_{10}\left(LT\right)}=\dfrac{0,4}{2}=0,2\left(mol\right)\\ n_{C_4H_{10}\left(TT\right)}=0,2:50\%=0,4\left(mol\right)\\ m_{C_4H_{10}\left(tt\right)}=58.0,4=23,2\left(g\right)\)

\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\\ a,PTHH:Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\\ n_{H_2}=n_{\left(CH_3COO\right)_2Mg}=n_{Mg}=0,2\left(mol\right);n_{CH_3COOH}=2.0,2=0,4\left(mol\right)\\ b,V_{H_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\\ c,m_{\left(CH_3COO\right)_2Mg}=142.0,2=28,4\left(g\right)\\ d,m_{ddCH_3COOH}=\dfrac{0,4.60.100}{12}=200\left(g\right)\)

\(n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\\ a,PTHH:Zn+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Zn+H_2\\ b,n_{H_2}=n_{\left(CH_3COO\right)_2Zn}=n_{Zn}=0,3\left(mol\right);n_{CH_3COOH}=2.0,3=0,6\left(mol\right)\\ b,V_{H_2\left(đkc\right)}=24,79.0,3=7,437\left(l\right)\\ c,m_{\left(CH_3COO\right)_2Zn}=0,3.183=54,9\left(g\right)\\ d,C_2H_5OH+CH_3COOH\rightarrow CH_3COOC_2H_5+H_2O\\ n_{Este\left(LT\right)}=n_{CH_3COOH}=0,6\left(mol\right)\\ n_{este\left(TT\right)}=80\%.0,6=0,48\left(mol\right)\\ m=m_{este\left(TT\right)}=88.0,48=42,24\left(g\right)\)

2CH3COOH + Zn -- > (CH3COOH)2Zn + H2
nH2 = 2,24 / 22,4 = 0,1 (mol)
=> nCH3COOH = 0,2 (mol)
mZn = 0,1. 65 = 6,5 (g)
mH2 = 0,1.2 = 0,2 (g)
mdd = 300 + 6,5 - 0,2 = 306,3 (g)
mCH3COOH = 0,2 . 60 = 12 (g)
=> C%CH3COOH = ( 12.100 ) / 306,3 = 4%
m(CH3COO)2Zn = 0,1 . 183 = 18,3 (g)
=> (18,3.100) / 306,3 = 6%

\(n_{HCl}=\dfrac{18.25}{36.5}=0.5\left(mol\right)\)
\(a.Mg+2HCl\rightarrow MgCl_2+H_2\)
\(b.\)
\(n_{Mg}=n_{H_2}=\dfrac{1}{2}\cdot n_{HCl}=\dfrac{1}{2}\cdot0.5=0.25\left(mol\right)\)
\(m_{Mg}=0.25\cdot24=6\left(g\right)\)
\(V_{H_2}=0.25\cdot22.4=5.6\left(l\right)\)
\(c.\)
\(V_{H_2\left(tt\right)}=5.6\cdot90\%=5.04\left(l\right)\)

a) PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
b) Ta có: \(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)=n_{MgCl_2}\)
\(\Rightarrow m_{MgCl_2}=0,15\cdot95=14,25\left(g\right)\)
c) Theo PTHH: \(\left\{{}\begin{matrix}n_{HCl\left(p.ứ\right)}=0,3\left(mol\right)\\n_{H_2}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{HCl\left(p.ứ\right)}=0,3\cdot36,5=10,95\left(g\right)\\m_{H_2}=0,15\cdot2=0,3\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Mg}+m_{ddHCl}-m_{H_2}=53,3\left(g\right)\)
\(\Rightarrow m_{ddHCl}=50\left(g\right)\) \(\Rightarrow C\%_{HCl\left(p.ứ\right)}=\dfrac{10,95}{50}\cdot100\%=21,9\%\)

a) n Zn = 6,5/65 = 0,1(mol)
Zn + 2CH3COOH $\to$ (CH3COO)2Zn + H2
Theo PTHH :
n CH3COOH = 2n Zn =0,2(mol)
C% CH3COOH = 0,2.60/200 .100% = 6%
b) n H2 = n Zn = 0,1(mol)
=> m dd sau pư = 6,5 + 200 - 0,1.2 = 206,3 gam
Theo PTHH : n (CH3COO)2Zn = n Zn = 0,1(mol)
=> C% (CH3COO)2Zn = 0,1.183/206,3 .100% = 8,87%
c)
C2H5OH + O2 $\xrightarrow{men\ giấm}$ CH3COOH + H2O
n C2H5OH pư = n CH3COOH = 0,2(mol)
=> m C2H5OH cần dùng = 0,2.46/80% = 11,5 gam
a) nZn=0,1(mol)
PTHH: Zn + 2 CH3COOH -> (CH3COO)2Zn + H2
0,1_______0,2_________0,1_____________0,1(mol)
mCH3COOH=0,2.60=12(g)
=> C%ddCH3COOH=(12/200).100=6%
b) mdd(CH3COO)2Zn= 6,5+200-0,1.2=206,3(g)
m(CH3COO)2Zn= 183 x 0,1=18,3(g)
=>C%dd(CH3COO)2Zn= (18,3/206,3).100=8,871%
c) C2H5OH + O2 -men giấm-> CH3COOH + H2O
nC2H5OH(LT)=nCH3COOH=0,2(mol)
=> nC2H5OH(TT)=0,2 : 80%= 0,25(mol)
=>mC2H5OH=0,25 x 46= 11,5(g)

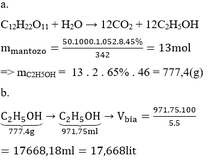

\(n_{BaCO_3}=\dfrac{19,7}{197}=0,1\left(mol\right)\\ a,PTHH:BaCO_3+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Ba+CO_2+H_2O\\ n_{CO_2}=n_{\left(CH_3COO\right)_2Ba}=n_{BaCO_3}=0,1\left(mol\right)\\ b,V_{CO_2\left(đktc\right)}=0,1.22,4=2,24\left(l\right)\\ c,m_{\left(CH_3COO\right)_2Ba}=255.0,1=25,5\left(g\right)\\ d,C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\\ n_{CH_3COOH}=0,1.2=0,2\left(mol\right);n_{C_2H_5OH\left(LT\right)}=n_{CH_3COOH}=0,2\left(mol\right)\\ n_{C_2H_5OH\left(TT\right)}=0,2.75\%=0,15\left(mol\right)\\ m_{C_2H_5OH\left(TT\right)}=0,15.46=6,9\left(g\right)\)