hoà tan aluminium dư bằng 500 (ml) dung dịch H2SO4 thì thu được 7, 437 (l) khí H2
a) tính nồng độ môl của dung dịch acid
b) tính khối lượng aluminium đã phản ứng
c) tính khối lượng muối sinh ra
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

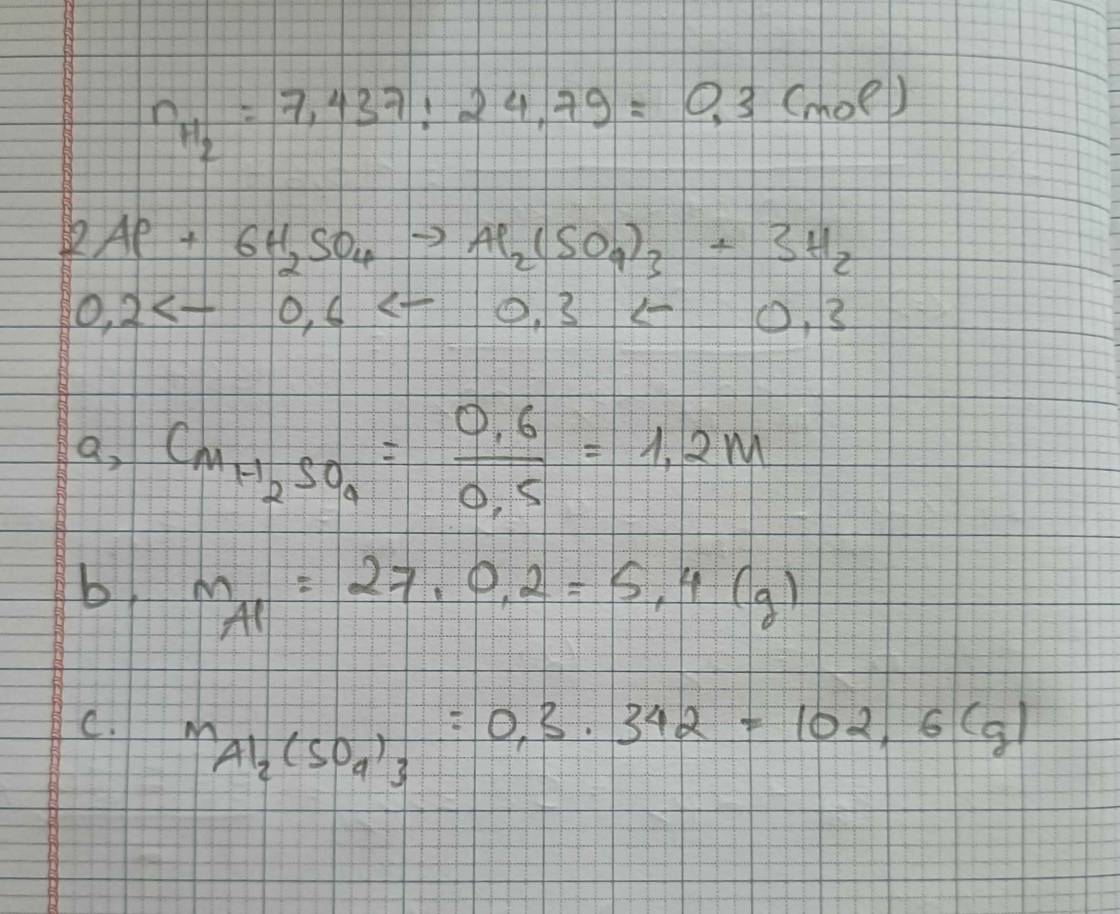

\(n_{H_2}=\dfrac{3,7185}{24,79}=0,15\left(mol\right)\)
PTHH: 2Al + 6HCl ---> 2AlCl3 + 3H2
0,1<---------------0,1<-----0,15
\(\Rightarrow\left\{{}\begin{matrix}a,m_{Al}=0,1.27=2,7\left(g\right)\\b,m_{AlCl_3}=0,1.133,5=13,35\left(g\right)\end{matrix}\right.\)

\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ 0,2........0,3...........0,1...........0,3\left(mol\right)\\ a.C_{MddH_2SO_4}=\dfrac{0,3}{0,3}=1\left(M\right)\\ b.m_{Al_2\left(SO_4\right)_3}=342.0,1=34,2\left(g\right)\\ c.V_{H_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\)
PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
a) \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{3}{2}n_{Al}=0,3\left(mol\right)\)
\(\Rightarrow C_{M_{H_2SO_4}}=\dfrac{0,3}{0,3}=1M\)
b) \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al_2\left(SO_4\right)_3}=n.M=0,1.342=34,2\left(g\right)\)
c) \(n_{H_2}=n_{H_2SO_4}=0,3\left(mol\right)\)
\(\Rightarrow V_{H_2}=n.22,4=0,3.22,4=6,72\left(l\right)\)

PT: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
Ta có: \(n_{H_2}=\dfrac{3,7185}{24,79}=0,15\left(mol\right)\)
a, \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,1\left(mol\right)\Rightarrow m_{Al}=0,1.27=2,7\left(g\right)\)
b, \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{3}n_{H_2}=0,05\left(mol\right)\)
\(\Rightarrow m_{Al_2\left(SO_4\right)_3}=0,05.342=17,1\left(g\right)\)
c, \(n_{H_2SO_4}=n_{H_2}=0,15\left(mol\right)\)
\(\Rightarrow V_{H_2SO_4}=\dfrac{0,15}{1,5}=0,1\left(l\right)=100\left(ml\right)\)

\(4.a/n_{Al}=\dfrac{5,4}{27}=0,2mol\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,2 0,3 0,1 0,3
\(V_{H_2}=0,3.24,79=7,437l\\ b/C_{\%H_2SO_4}=\dfrac{0,3.98}{150}\cdot100=19,6\%\\ c/m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2g\)
\(5.a/n_{MgO}=\dfrac{4}{40}=0,1mol\\ MgO+2HCl\rightarrow MgCl_2+H_2O\)
0,1 0,2 0,1 0,1
\(C_{\%HCl}=\dfrac{0,2.36,5}{200}\cdot100=3,65\%\\ b/C_{\%MgCl_2}=\dfrac{0,1.95}{200+4}\cdot100=4,66\%\\ c/NaOH+HCl\rightarrow NaCl+H_2O\\ n_{NaOH}=n_{HCl}=0,2mol\\ V_{NaOH}=\dfrac{0,2}{1}=0,2l=200ml\)

\(n_{Al}=\dfrac{2,7}{27}=0,1mol\\ a.2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,1 0,15 0,05 0,15
\(b.V_{H_2}=0,15.24,79=3,7185l\\ c.m_{Al_2\left(SO_4\right)_3}=0,05.342=17,1g\\ d.C_{M_{H_2SO_4}}=\dfrac{0,15}{0,4}=0,375M\)

a) 2Al+6HCl→→2AlCl3+3H2
b)
nAl=10,8\27=0,4(mol)
nAlCl3=nAl=0,4(mol)
mAlCl3=0,4.133,5=53,4(g)
c)
nH2=3\2nAl=0,6(mol)
VH2=22,4.0,6=13,44(l)
d) n HCl=0,4.6\2=1,2 mol
=>Cm HCl=1,2\0,1=12M
\(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
a) Pt : \(2Al+6HCl\rightarrow2AlCl_3+3H_2|\)
2 6 2 3
0,4 1,2 0,4 0,6
b) \(n_{H2}=\dfrac{0,4.3}{2}=0,6\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,6.24,79=14,874\left(l\right)\)
c) \(n_{AlCl3}=\dfrac{0,6.2}{3}=0,4\left(mol\right)\)
⇒ \(m_{AlCl3}=0,4.133,5=53,4\left(g\right)\)
d) \(n_{HCl}=\dfrac{0,4.6}{2}=1,2\left(mol\right)\)
100ml = 0,1l
\(C_{M_{ddHCl}}=\dfrac{1,2}{0,1}=12\left(M\right)\)
Chúc bạn học tốt

\(a/2Al+3H_2SO_4\xrightarrow[]{}Al_2\left(SO_4\right)_3+3H_2\)
\(b/30ml=0,03l\\ n_{H_2SO_4}=0,5.0,03=0,0015\left(mol\right)\\ n_{Al}=\dfrac{0,0015.2}{3}=0,001\left(mol\right)\\ m_{Al}=0,001.27=0,027\left(g\right)\\ n_{Al_2\left(SO_4\right)_3}=\dfrac{0,0015}{2}=0,00075\left(mol\right)\\ m_{Al_2\left(SO_4\right)_3}=0,00075.342=0,2565\left(g\right)\)
\(c/n_{H_2}=\dfrac{0,0015.3}{3}=0,0015\left(mol\right)\\ V_{H_2}=0,0015.24,79=0,037185\left(l\right)\)
\(a.2Al+3H_2SO_4->Al_2\left(SO_4\right)_3+3H_2\\ b.n_{Al}=1,5.0,5.0,03=0,0375mol\\ m_{Al}=0,0375.27=1,0125g\\ m_{Al_2\left(SO_4\right)_3}=342\cdot\dfrac{1}{3}\cdot0,03\cdot0,5=1,71g\\V_{H_2}=24,79.0,5.0,03=0,37185L\)

a)
$n_{Al} = 0,3(mol)$
$2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2$
Theo PTHH :
$n_{H_2SO_4} = \dfrac{3}{2}n_{Al} = 0,45(mol)$
$m_{dd\ H_2SO_4} = \dfrac{0,45.98}{12,25\%} = 360(gam)$
b)
$n_{H_2} = n_{H_2SO_4} = 0,45(mol)$
$V_{H_2} = 0,45.22,4 = 10,08(lít)$
c)
$n_{Al_2(SO_4)_3} = 0,15(mol)$
$m_{dd\ sau\ pư} = 8,1 + 360 - 0,45.2 = 367,2(gam)$
$C\%_{Al_2(SO_4)_3} = \dfrac{0,15.342}{367,2}.100\% = 14\%$

